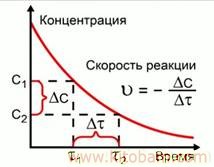
SURATI REAKSIYaI KhIMIYaVI, mafhumi asosii kinetikai khimiyavist, ki tagyir yoftani adadi zarraho (molekulaho, ionho)-i hamaguna moddai dar reaksiya ishtirokkunandaro dar vohidi vaqt ba vohidi hajm (baroi reaksiyahoi gomogeni) yo ba vohidi masohati sath (baroi reaksiyahoi geterogeni va topokhimiyavi) ifoda mekunad. Surati reaksiyahoi khimiyaviro miqdoran az rui tagyiri konsentrasiyai moddahoi ba reaksiya dokhilshavanda yo mahsuli reaksiya muayyan mekunand. Molekulaho dar vohidi vaqt har qadar ziyodtar ba ham barkhurand, Surati reaksiyahoi khimiyavi hamon qadar ziyod meshavad. Hangomi baland shudani harorat surati harakati molekulaho ziyod shuda, sababi afzudani adadi molekulahoi ba ham barkhuranda megardad. Barobari in Surati reaksiyahoi khimiyavi ziyod meshavad. Hangomi baland shudani harorat ba har 10°S Surati reaksiyahoi khimiyavi gomogeni mutanosiban 2—4 marotiba meafzoyad (qonuni Vant — Goff). Surati reaksiyahoi khimiyaviro inchunin bo katalizatorho niz tagyir dodan mumkin ast. Surati tabadduloti gunoguni khimiyavi dar tabiat har khel ast. Masalan, khodisahoi tarkish varadu barq (az dah hazor yak qismi soniya), hodisai zangzanii metallho va masnuoti metalli, ba vujud omadani mineralho va gayra dahho, sadho va hazorho sol davom mekunand. Holo insoniyat sharoiti bavujudoii reaksiyahoi khimiyaviro ba hisob girifta, surati onhoro dar sanoat tagyir doda, baroi samaranokii mahsuloti khimiyavi, peshrafti khojagii khalq istifoda mebarand. Surati reaksiyahoi khimiyaviro bo usulhoi fotometri, polirografi, potensiometri va gayra muaiyan mekunand.
Adabiyot: Emanuel N. M., Knorre D.G. Kurs khimicheskoy kinetiki, Moskva, 1974; Glinka N. L., Obshaya khimiya, Leningrad. 1977. N. Qobilov.
 Таджикистан – Энциклопедия на таджикском языке КИТОБАМ
Таджикистан – Энциклопедия на таджикском языке КИТОБАМ